The safety of Clostridium butyricum
- Categories:News Center
- Author:
- Origin:PUBLIC VERSION - CLOSTRIDIUM BUTYRICUM MIYAIRI 588 NOVEL FOOD APPLICATION
- Time of issue:2022-03-28 15:20
- Views:
The safety of Clostridium butyricum
- Categories:News Center
- Author:
- Origin:PUBLIC VERSION - CLOSTRIDIUM BUTYRICUM MIYAIRI 588 NOVEL FOOD APPLICATION
- Time of issue:2022-03-28 15:20
- Views:
Over 500 different species of micro-organisms inhabit the human gut, some of which are harmless or beneficial to the host and others potentially harmful (Tuohy et al 2003). Clostridium is a large bacterial genus with more than 150 species (Durre 2009; Hippe et al 2002), all gram positive, spore-forming bacteria, obligate anaerobes. Most scientists are aware of the pathogenic Clostridium species, notably Clostridium botulinum, Clostridium difficile, Clostridium perfringens and Clostridium tetani (Smith 1992). However, only a few members of this genus, less than 10%, form dangerous toxins (Durre 2009). Most clostridial species, especially gut-associated clostridial species such as Clostridium butyricum, are non-pathogenic gut commensals, which dominate and form an important part of the lower gut flora of both man and animals (Finegold et al 1983). The importance of clostridial species as a major bacterial component in the normal gut flora of all mammals and birds had been underestimated until the advent of modern molecular microbiological methods, possibly due to historical difficulties of cultivation in vitro, associated with the requirements of the genus Clostridium for strict anaerobic conditions.
Nowadays it is appreciated that after birth, the infant intestine is progressively colonised by facultative and strictly anaerobic bacteria, including bacteria from the Clostridium genus (Mountzouris et al 2002). Clostridium butyricum was first isolated and characterised by Prazmowski in 1880 (cited by Durre 2009), and may be found in the intestinal content of healthy humans, colonising the colon of infants soon after birth and forming part of the dominant, non- pathogenic, commensal clostridial group. Surveys have identified Clostridium butyricum in 20% of human faecal samples by microbial culture. Higher detection rates are noted when intestinal contents are sampled and strict anaerobiosis is maintained from sampling to culture (Finegold et al 1983; Sato & Tanaka 1997). A recent UK study (Ghoddusi & Sherburn 2010) yielded presumptive Clostridium butyricum strains from 302 of 978 environmental samples tested (31%). The highest percentage of positive isolations came from soil, potato skins, Swede skin, yoghurt and cream. Early blowing by Clostridium butyricum of Grana cheese has been reported (Bottazzi 2001, cited by Quiberoni et al 2008). In these respects Clostridium butyricum is not novel to the human gut, since it may be consumed with common food products and it forms a natural part of the gut flora of a significant proportion of human infants, children and adults, as well as being a common environmental commensal.
Clostridium butyricum MIYAIRI 588 (CBM 588) is a wild strain of Clostridium butyricum, originally isolated from a soil sample in Nagano, Japan, in 1963 and deposited in 1972 at the National Institute of Advanced Industrial Science and Technology, International Organism Depository (AIST) in Japan. Clostridium butyricum FERM-BP 2789 is the current strain deposition number for CBM 588. The strain has not been modified genetically.
Miyarisan has sold preparations of CBM 588 in Japan and other Asian countries for both human and animal use since the 1960s with no adverse events or reports of allergenicity (Confidential Annex 2). Published in vitro and in vivo data and a long history of safe use in humans in Asian countries help support CBM 588 as a safe strain for use in food supplements in the EU (e.g. Confidential Annex 4; Fujita et al 1986; Fujita & Takashi, 1986, 1987; Imase et al 2008; Ito et al 1997; Kamiya et al 1997; Kitajo et al 1990, 1991; Kobashi et al 1983, 1976; Kobayashi et al 1976; Kurata et al 1988; Kuroiwa et al 1990a, 1990b; Maebashi et al 1998; Nakatsuji et al 1995; Okabayashi & Kobari 1994; Seki et al 2003; Shimbo et al 2005; Taguchi et al 1988; Takahashi et al 2000, 2004; Takeda et al 1976, 1983, Yuzawa et al 1987a, b, c; Zhang et al 1998).
In 2009, a preparation based on CBM 588 was approved in the EU as a zootechnical (functional group: gut flora enhancer) feed additive for fattening chickens and in 2011 for weaned piglets, minor avian and porcine species (Com. Reg. 903/2009; Com. Reg. 373/2011; EFSA 2009, EFSA 2011a).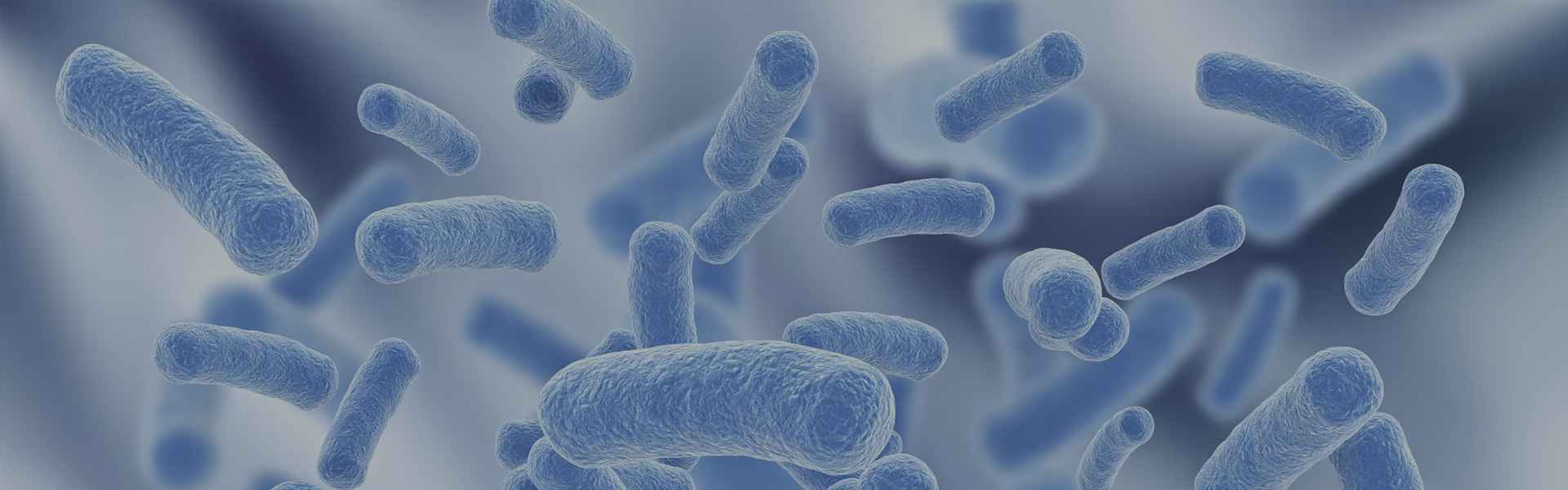
Service Hotline:
Tel:+86-371-61779068
Phone:+86-18602468822
WhatsApp:+86-15717345927
Skype: +86-18639087551
Email:melody@jbh2013.com
Add: Fudao Town, tangyin county, Anyang City, Henan Province
Copyright © 2020 Henan JBH Bio-Tech Co., Ltd 豫ICP备15035929号 Business License Powered by : www.300.cn SupportedIPV6

